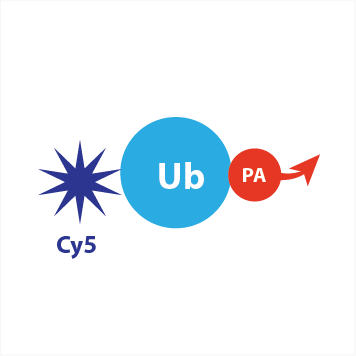
Cy5-Ub-PA
fluorescent activity-based probe for deubiquitinating enzymes (DUBs)
Additional information
| Weight | 0.005 kg |
|---|---|
| aliquot size | |
| Applications | Crystallization, Pull down, Purification, Western Blot, Phenotypic protein profiling |
| target | |
| source | |
| shipping | |
| purity | |
| molecular weight | |
| storage | Powder at −20°C; solution at −80°C. Please avoid multiple freeze/thaw cycles. |
| sample preparation | For detailed sample preparation see product sheet. |
| regulatory statement |
€275.00
- Description
- Additional information
- references
Description
UbiQ-072 is an activity-based probe for deubiquitinating enzymes (DUBs). It is based on ubiquitin functionalised with a C-terminal propargyl amide (PA) and N-terminal Cy5 dye. It can be used for activity profiling experiments and determining DUB inhibitor specificity.
- the PA group forms a covalent linkage with (the active site Cys residue of) a DUB that can be cleaved by acid treatment (5% aq. TFA), allowing for proteomic analyses;
- the PA group targets all three major DUB families: UCH, USP and OTU;
- the fluorescent label allows detection of DUB labeling by direct in-gel fluorescence. This is a less time-consuming and more sensitive read-out than western-blotting
- cross-reactivity of antibodies can lead to background labeling, something that is not observed with UbiQ-072
Additional information
| Weight | 0.005 kg |
|---|---|
| aliquot size | |
| Applications | Crystallization, Pull down, Purification, Western Blot, Phenotypic protein profiling |
| target | |
| source | |
| shipping | |
| purity | |
| molecular weight | |
| storage | Powder at −20°C; solution at −80°C. Please avoid multiple freeze/thaw cycles. |
| sample preparation | For detailed sample preparation see product sheet. |
| regulatory statement |
de Jong et al.
de Jong, A., et al. Ubiquitin-based probes prepared by total synthesis to profile the activity of deubiquitinating enzymes. ChemBiochem 13, 2251-2258 (2012).
http://www.ncbi.nlm.nih.gov/pubmed/23011887
Altun et al.
Altun, M., et al. Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem. Biol. 18, 1401-1412 (2011).
http://www.ncbi.nlm.nih.gov/pubmed/22118674
Ekkebus et al.
Ekkebus, R., et al. On terminal alkynes that can react with active-site cysteine nucleophiles in proteases. J. Am. Chem. Soc. 135, 2867-2870 (2013).
http://www.ncbi.nlm.nih.gov/pubmed/23387960
Sommer et al.
Sommer, S., et al. Covalent inhibition of SUMO and ubiquitin-specific cysteine proteases by an in situ thiol-alkyne addition. Bioorg. Med. Chem. 21, 2511-2517 (2013).
http://www.ncbi.nlm.nih.gov/pubmed/23535560
